Defining and Defending a Blood Pressure in the Operating Room & Intensive Care Unitby Ashish K. Khanna MD., FCCP., FCCM
Despite surgical patients presenting to the operating room (OR) sicker than ever before, the intraoperative period has, paradoxically, become far safer. Current estimates of intraoperative mortality are less than 1 in 100,000 patients (1). However, 30-day postoperative mortality is still significant at 1-2%, a fact that suggests that if 30-day postop mortality were considered a disease, it would be the third leading cause of death in the United States (2). There are few interventions that anesthesiologists and intensivists can offer that have direct evidence of improved patient survival. The one goal of anesthetic management over the years has been hemodynamic stability. There is now, more than ever before, direct evidence of the damaging effects of low blood pressure. These effects are clearly associated with myocardial injury, mortality and acute kidney injury. In addition, these effects extend beyond the operating room, into the post-surgical recovery wards and for the critically ill patient in the intensive care unit (ICU). Appropriate definition of threshold pressures in the operating room and ICU and a rationale strategy for defending these is of utmost importance in current practice. Intraoperative Blood Pressure Randomized trials of intra-operative blood pressure control are rare and difficult to conduct. Notably, recent work by the INPRESS investigators lead by Futier and colleagues compared tight vs. minimal intraoperative blood pressure control (n=298).(7) High-risk patients were randomized to minimal blood pressure control (ephedrine for systolic pressure <80 mmHg or <40% below baseline) vs. a norepinephrine infusion to maintain systolic pressure within 10% of baseline values during and for four hours after surgery. The primary outcome, a composite of systemic inflammatory response syndrome and/or at least one organ failure, occurred in 56/147 patients in the norepinephrine (tight control) group vs 75/145 patients in the minimal control group: relative risk 0.73 (95% CI: 0.56, 0.94). The intervention threshold of a systolic pressure of 80 mmHg is low compared to what most anesthesiologists would react to in real life. It is very likely that a higher intervention pressure presumably would have reduced the observed 25% benefit. Blood Pressure in the Critically Ill Patient in Intensive Care Units Hypotension in critical care units may be profound, prolonged and especially damaging because of several co-existing, complicated and often cumulative other insults. Similar to the intraoperative period, there is increasing evidence for an association between hypotension at thresholds that seem to challenge the general norm of 65 mmHg established by the surviving sepsis campaign guidelines and serious complications. (8-10) At the Cleveland Clinic, we recently evaluated a cohort of 2,918 postoperative critical care patients and saw an association between the lowest recorded MAP and a primary composite of myocardial injury and in-hospital mortality at 7 days. At MAP less than 90 mmHg, every 10-mmHg difference between patients in the lowest MAP on any given day increased the risk of the primary composite by about 50%.(8) (Fig.3) Hypotension and acute kidney injury was linearly related over the entire range of MAP from 50 mmHg to 110 mmHg, with an adjusted overall hazard ratio of 1.16 (1.29 for stage 2&3 acute kidney injury) per 10-mmHg reduction in the lowest recorded MAP. We have also shown that both the duration and severity of hypotension matter. For example, an hour of exposure to 80 mmHg was similar in damage to even a few minutes below 70 mmHg.(8) Whereas these data were from a postoperative (mostly non-septic) patient cohort, another recent examination of nearly 9,000 septic patients across 110 ICUs in the United States saw a similar signal for harm at a higher threshold MAP. Specifically, this study saw that the risks for mortality, AKI, and myocardial injury first developed at a MAP of 85 mmHg, and the risk of mortality and AKI progressively worsened at lower blood pressures, on a MAP range from 55-85 mmHg.(Fig. 4)(9) Other smaller cohorts have also reported MAP-associated organ system injury at thresholds normally considered safe in the surgical and mixed ICU populations. (11) (12) (13) (14) It is worth keeping in mind, however, that the interpretation of all these hypotension data is complicated by sepsis. Because sepsis results in both hypotension and organ system injury from independent, although related, mechanisms, significant dependent-variable confounding occurs in all of these analyses.(15) Interestingly, hypotension in critical care units has also been associated with delirium. Aldemir and colleagues screened 818 critical care patients daily for 10 days and reported an association between systolic pressure <80 mmHg and delirium.(16) Likewise, at the Cleveland Clinic, we have seen that a 10-mmHg reduction in the lowest MAP on each day during ICU stay at a MAP of less than 65 mmHg was significantly associated with a higher hazard of delirium, with an adjusted hazard ratio 1.16 (95% CI: 1.07, 1.26; P<0.001) (unpublished data-Cleveland Clinic). Only one large multicenter randomized controlled trial of different blood pressure targets has been performed to date. In that study, no difference in mortality was note for blood pressure targets of 80-85 mmHg or 65-70 mmHg in patients with septic shock.(17) However, a closer look at this work shows that the actual blood pressure targets achieved were different (85-90 mmHg vs. 70-75 mmHg) from experimental design, making it difficult to answer questions about hypotension from this published work. In addition, there were no assessment of myocardial injury (MINS) based on systematic collection of troponin bioanalysis and clinical myocardial infarction were only observed in 9 patients which precluded reliable assessment of this important outcome. Other important findings from this landmark work were that atrial fibrillation was more common in patients assigned to higher blood pressure and patients with chronic hypertension who were assigned to the lower pressure target had more renal injury and the prevalence of renal replacement therapy was higher in this subgroup. This study and other smaller studies have significantly associated higher blood pressure targets with more cardiac arrhythmias and vasopressor use, without detecting a difference in serum lactate, regional blood flow, or mortality compared with lower blood pressure targets.(18-21) Conclusions Overall, we may be correct in defining a harm threshold of MAP of about 65 mmHg in the intraoperative period, and possibly considerably greater in critical care units; however, it also seems likely that this may not be an adequate threshold in all patient groups. The available data is largely retrospective and has several concerns for confounding. While this concise review covers the most recent work in this area, readers are also referred to a comprehensive overview of all contemporary published literature & relevant trials.(22) Randomized data is sparse, but suggests that at least some harm can be prevented by intervening to limit the severity and duration of even mild hypotension. The message remains loud & clear for Critical Care Anesthesiologists: “Define a MAP and then defend that MAP” whatever clinical setting your patient may be in. References
Figures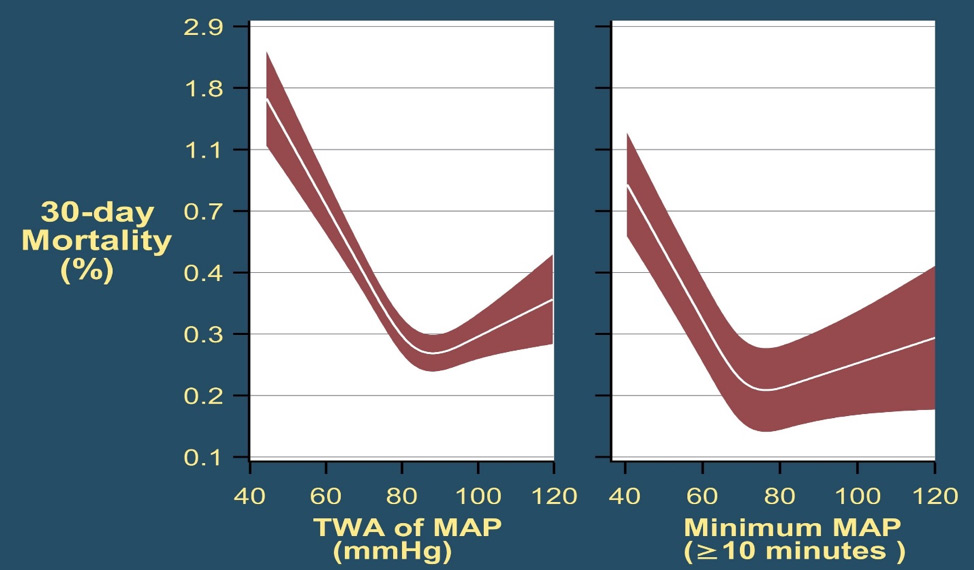 Fig. 1: Mortality in the 30 post-operative days as a function of time-weighted average (TWA) mean arterial pressure or lowest mean pressure maintained for at least 10 minutes. With permission from Mascha et al: Intraoperative mean arterial pressure variability and 30-day mortality in patients having noncardiac surgery. Anesthesiology 2015; 123: 79-91 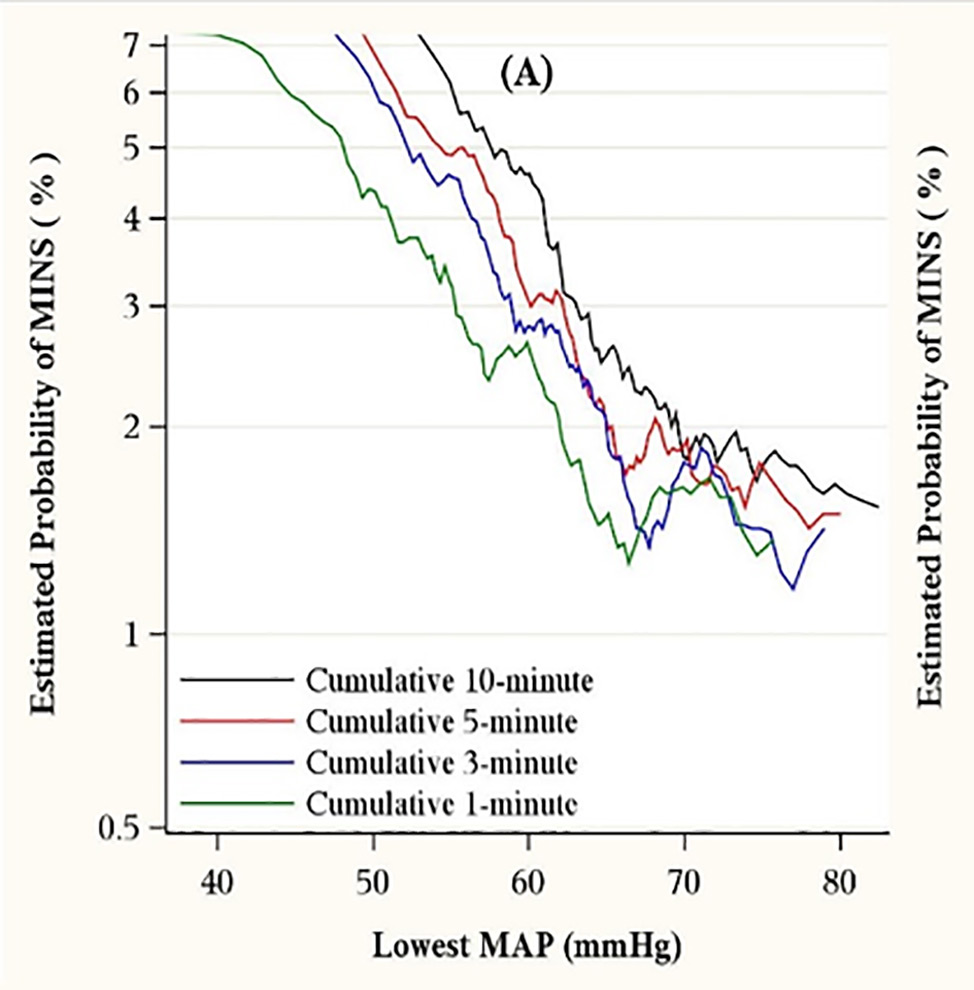 Fig. 2: The left graph (A) shows the relationship between the lowest cumulative absolute mean arterial pressure maintained for 1, 3, 5, and 10 minutes and myocardial injury. The right graph (C) shows the relationship between the lowest cumulative relative mean arterial pressure maintained for 1, 3, 5, and 10 minutes and myocardial injury. Both were highly predictive, but relative thresholds were not more predictive than absolute thresholds which are easier to use. The relationships were generally similar for acute kidney injury (not shown). With permission from Salmasi, et al: Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: A retrospective cohort analysis. Anesthesiology 2017; 126: 47-65. 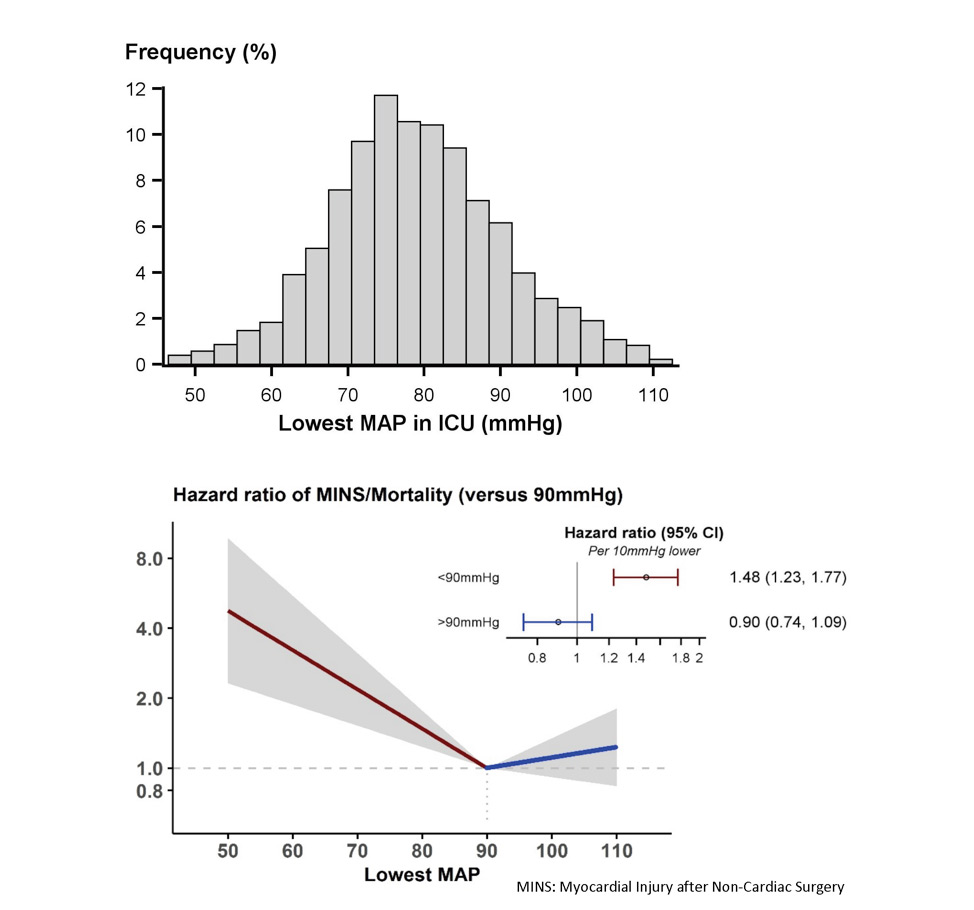 Fig. 3: Association of exposure to hypotension at mean arterial pressures (MAP) less than 90 mm Hg and a primary composite outcome of myocardial injury after non-cardiac surgery (MINS) and mortality. For any two postoperative patients in the surgical ICU with a 10 mm Hg difference in the lowest MAP per day of less than 90 mmHg there was a nearly 50% increase in the hazards of the primary outcome. With permission from Khanna AK et al: Hypotension increases acute kidney injury, myocardial injury and mortality in surgical critical care. Critical Care Medicine 2018, 46(1):71. (Presented at SCCM 2018, San Antonio, TX) 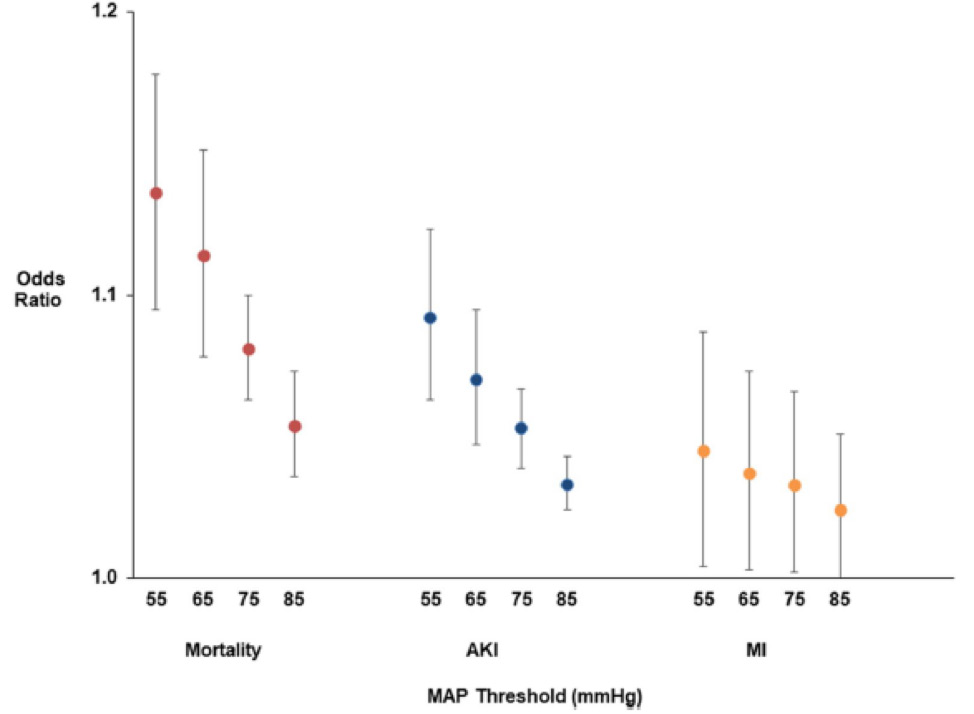 Fig. 4: Association of hypotension exposure with in-hospital mortality, AKI and myocardial injury. Adjusted odds ratios and 95% confidence intervals for a 1 mmHg increase in TWA-MAP, below different thresholds are shown for the primary outcome of in-hospital mortality and secondary outcomes of acute kidney injury and myocardial injury. With permission from Maheshwari, et al: The relationship between ICU hypotension and in-hospital mortality and morbidity in septic patients. Intensive Care Med. 2018 Jun 5. doi: 10.1007/s00134-018-5218-5. [Epub ahead of print] Author |

